Now available Vancomycin Hydrochloride for Oral Solution USP (GENERIC equivalent to Vancocin® for Oral Solution)
For Patients Who Need
Vancomycin In An Oral Solution
Why Vancomycin Hydrochloride for Oral Solution USP†
- Oral Solution dosage form is easier to swallow for younger patients and those with dysphagia
- Pre-flavored convenience with a Mixed Berry taste
- Improved dosing flexibility with multiple bottle sizes available
- Ability to administer via nasogastric tube
- Contains no ingredient made from a gluten-containing grain (wheat, barley, or rye)
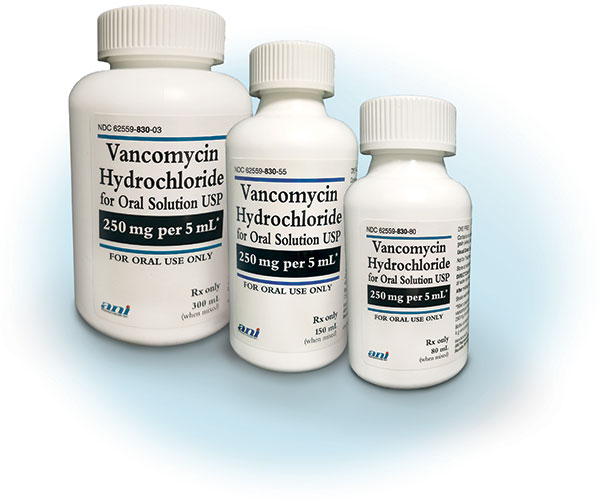
How supplied (250 mg per 5 mL*):
Swipe Chart To Scroll Left / Right
| Reconstitution Volume | Total Content‡ | National Drug Code (NDC) | Common Use |
|---|---|---|---|
| 80 mL | 4,000 mg | 62559-830-80 | Tapering or post hospital discharge |
| 150 mL | 7,500 mg | 62559-830-55 | Most common |
| 300 mL | 15,000 mg | 62559-830-03 | For patients needing 14-day therapy |
Single bottle storage
~ Simple reconstitution
~ Mixed Berry taste
DYE-FREE
~ Tapering options
~ Refrigeration required
Swipe Chart To Scroll Left / Right
| National Drug Code (NDC) |
Amerisource Bergen |
Cardinal | McKesson |
|---|---|---|---|
| 62559-830-80 | 10229903 | 5564554 | 3984309 |
| 62559-830-55 | 10229900 | 5564562 | 3984325 |
| 62559-830-03 | 10229901 | 5564570 | 3984317 |
For additional information, please call 1-800-434-1121 or visit www.anipharmaceuticals.com.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
* When prepared as directed, each 5 mL of solution contains vancomycin hydrochloride equivalent to approximately 250 mg of vancomycin.
† Vancomycin Hydrochloride for Oral Solution USP [package insert]. Baudette, MN: ANI Pharmaceuticals, Inc.
‡ Content of vancomycin per bottle.
Vancocin® is a registered trademark of ANI Pharmaceuticals, Inc.
